15. Chemical Kinetics
Rate Law
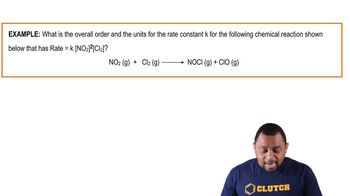
Problem 32b
Textbook Question
Textbook QuestionThe react ion between ethyl bromide 1C2H5Br2 and hydroxide ion in ethyl alcohol at 330 K, C2H5Br1alc2 + OH- 1alc2¡ C2H5OH1l2 + Br - 1alc2, is first order each in ethyl bromide and hydroxide ion. When 3C2H5Br4 is 0.0477 M and 3OH- 4 is 0.100 M, the rate of disappearance of ethyl bromide is 1.7 * 10-7 M>s. (a) What is the value of the rate constant?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
374
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos