6. Chemical Quantities & Aqueous Reactions
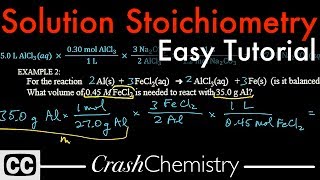
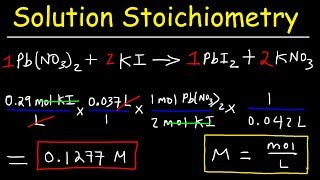
Solution Stoichiometry
Problem 92
Textbook Question
Textbook QuestionThe following three solutions are mixed: 100.0 mL of 0.100 M Na2SO4, 50.0 mL of 0.300 M ZnCl2, and 100.0 mL of 0.200 M Ba(CN)2. (b) What is the molarity of each ion remaining in the solution assuming complete precipitation of all insoluble compounds?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
994
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos