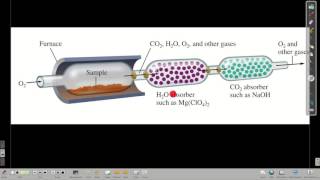
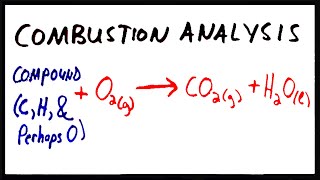
3. Chemical Reactions
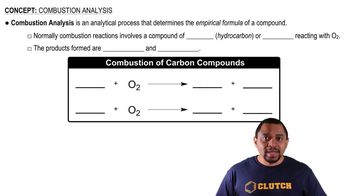
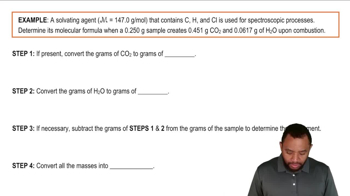
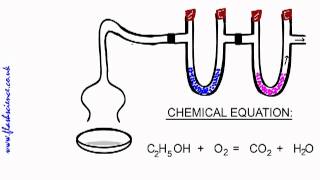
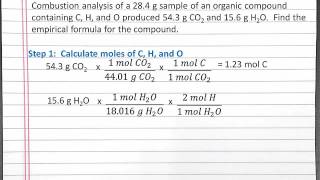
Combustion Analysis
Problem 55c
Textbook Question
Textbook Question(a) Combustion analysis of toluene, a common organic solvent, gives 5.86 mg of CO2 and 1.37 mg of H2O. If the compound contains only carbon and hydrogen, what is its empirical formula?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1408
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos