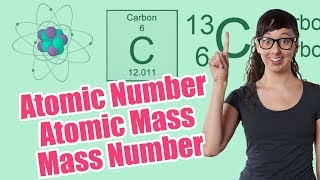2. Atoms & Elements
Atomic Mass
Problem 116
Textbook Question
Textbook QuestionBoron has only two naturally occurring isotopes. The mass of boron-10 is 10.01294 amu and the mass of boron-11 is 11.00931 amu. Calculate the relative abundances of the two isotopes.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
8134
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos