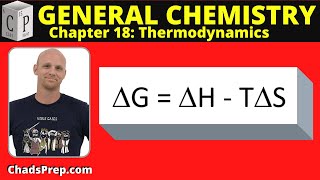
19. Chemical Thermodynamics
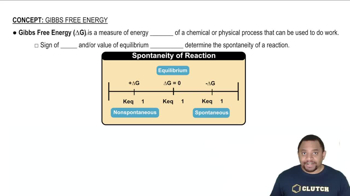
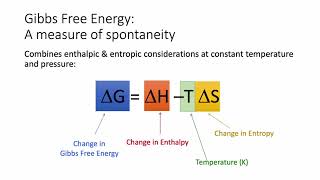
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 122
Textbook Question
Textbook QuestionUse the data in Appendix B to calculate the equilibrium pressure of CO2 in a closed 1 L vessel that contains each of the following samples:
(a) 15 g of MgCO3 and 1.0 g of MgO at 25 °C
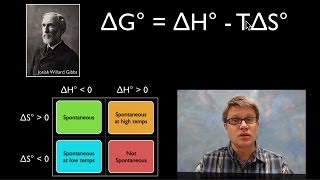
Assume that ∆H° and ∆S° are independent of temperature.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
165
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos