3. Chemical Reactions
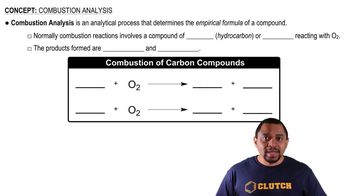
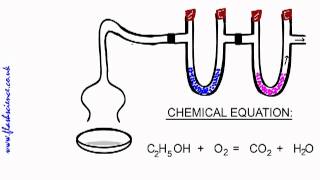
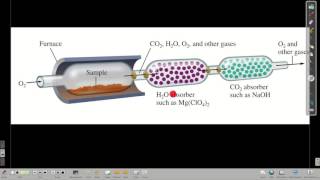
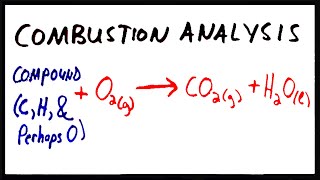
Combustion Analysis
Problem 55a
Textbook Question
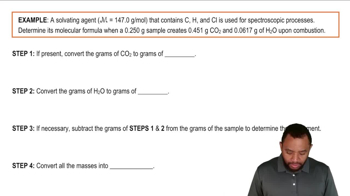
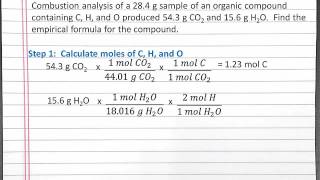
Textbook Question(b) Menthol, the substance we can smell in mentholated cough drops, is composed of C, H, and O. A 0.1005-g sample of menthol is combusted, producing 0.2829 g of CO2 and 0.1159 g of H2O. What is the empirical formula for menthol? If menthol has a molar mass of 156 g/mol, what is its molecular formula?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
1694
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos