7. Gases
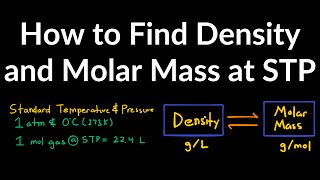
The Ideal Gas Law: Molar Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 53
Textbook Question
Textbook QuestionIn the Dumas-bulb technique for determining the molar mass of an unknown liquid, you vaporize the sample of a liquid that boils below 100 °C in a boiling-water bath and determine the mass of vapor required to fill the bulb. From the following data, calculate the molar mass of the unknown liquid: mass of unknown vapor, 1.012 g; volume of bulb, 354 cm3; pressure, 98.93 kPa; temperature, 99 °C.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
892
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos