12. Molecular Shapes & Valence Bond Theory
MO Theory: Bond Order
Problem 75b
Textbook Question
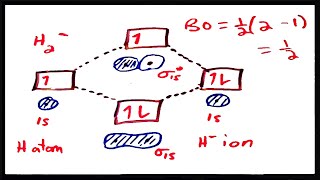
Textbook QuestionUsing the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at lower energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? c. 8
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
360
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos