7. Gases
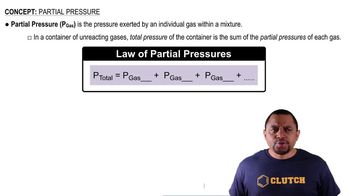
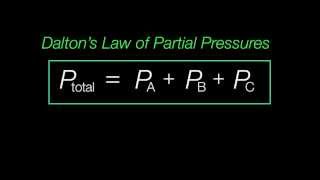
Partial Pressure
Problem 68
Textbook Question
Textbook QuestionA sample of 5.00 mL of diethylether 1C2H5OC2H5, density = 0.7134 g>mL2 is introduced into a 6.00-L vessel that already contains a mixture of N2 and O2, whose partial pressures are PN2 = 21.08 kPa and PO2 = 76.1 kPa. The temperature is held at 35.0 °C, and the diethylether totally evaporates. (b) Calculate the total pressure in the container.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
973
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos