12. Molecular Shapes & Valence Bond Theory
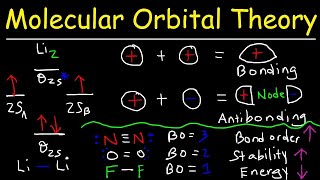
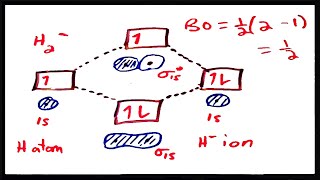
MO Theory: Bond Order

Get help from an AI Tutor
Ask a question to get started.
Problem 112
Textbook Question
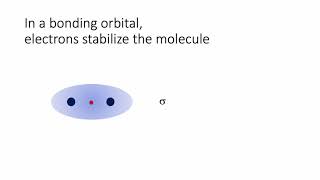
Textbook QuestionThe energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding π orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the p2p to the p*2p molecular orbital. (c) Is the C¬C bond in ethylene stronger or weaker in the excited state than in the ground state? Why?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
366
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos