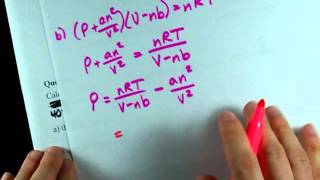7. Gases
Van der Waals Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 91
Textbook Question
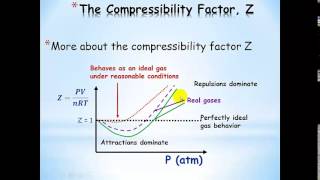
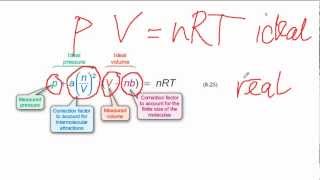
Textbook QuestionWhich statement concerning the van der Waals constants a and b is true? (a) The magnitude of a relates to molecular volume, whereas b relates to attractions between molecules. (b) The magnitude of a relates to attractions between molecules, whereas b relates to molecular volume. (c) The magnitudes of a and b depend on pressure. (d) The magnitudes of a and b depend on temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1530
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos