8. Thermochemistry
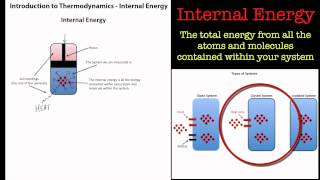
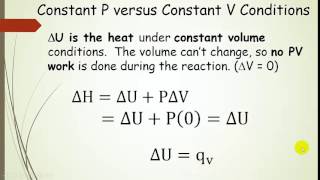
Internal Energy
Problem 37b
Textbook Question
Textbook QuestionWhich statement is true of the internal energy of a system and its surroundings during an energy exchange with a negative ΔEsys? a. The internal energy of the system increases and the internal energy of the surroundings decreases. b. The internal energy of both the system and the surroundings increases. c. The internal energy of both the system and the surroundings decreases. d. The internal energy of the system decreases and the internal energy of the surroundings increases.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
2158
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos