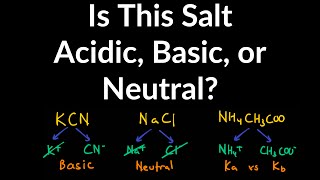
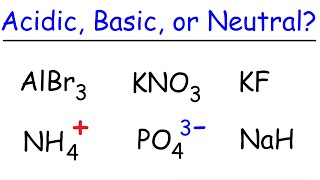
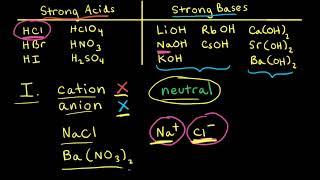
17. Acid and Base Equilibrium
Ionic Salts
Problem 64a
Textbook Question
Textbook QuestionAn element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (b) Do you think that element X is a metal, nonmetal, or metalloid?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
284
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos