Isotopes are variations of elements that share the same number of protons but differ in the number of neutrons. Understanding isotopes introduces key concepts such as atomic number and mass number, which are essential for identifying elements and their properties. The atomic number, denoted by the variable z, indicates the number of protons in an atom. This number is crucial because it allows us to identify an element on the periodic table, thus revealing its identity and potential chemical properties.
In addition to the atomic number, the mass number, represented by the variable a, is the total count of protons and neutrons in an atom. To find the number of neutrons in an isotope, one can use the formula:
Number of Neutrons = Mass Number (a) - Atomic Number (z)
Atoms consist of four primary components: the nucleus, which contains protons and neutrons, and electrons that orbit around the nucleus. For example, if an atom has 5 protons and 6 neutrons, the mass number would be calculated as follows:
Mass Number (a) = Number of Protons + Number of Neutrons = 5 + 6 = 11
This mass number is often represented as a purple symbol a in diagrams. In this case, with 5 protons, the atomic number is also 5, which corresponds to the element boron on the periodic table.
It is important to note that in a neutral atom, the number of protons equals the number of electrons, resulting in no overall charge. This balance of positive and negative charges leads to a neutral element. In contrast, ions are species where the number of protons and electrons is unequal, resulting in a net charge. For now, understanding the relationship between protons, neutrons, and electrons is fundamental to grasping the structure and identity of atoms.


 ?
?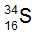 ?
?