10. Periodic Properties of the Elements
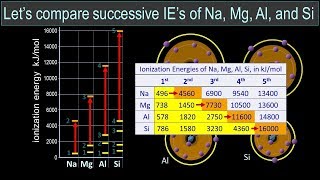
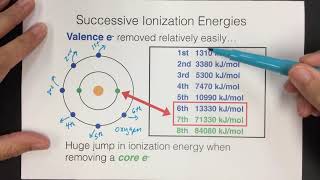
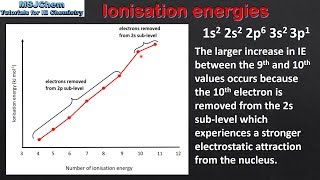
Periodic Trend: Successive Ionization Energies
Problem 32
Textbook Question
Textbook QuestionDeuterium oxide 1D2O, where D is deuterium, the hydrogen-2 isotope) has an ion-product constant, Kw, of 8.9 * 10-16 at 20 °C. Calculate 3D+4 and 3OD-4 for pure (neutral) D2O at this temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
2262
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos