8. Thermochemistry
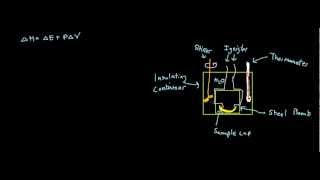
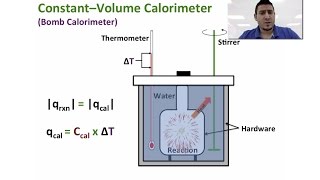
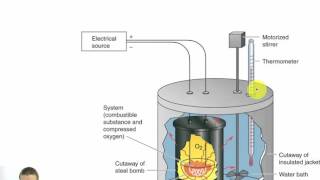
Constant-Volume Calorimetry
Problem 71
Textbook Question
Textbook QuestionExactly 1.5 g of a fuel burns under conditions of constant pressure and then again under conditions of constant volume. In measurement A the reaction produces 25.9 kJ of heat, and in measurement B the reaction produces 23.3 kJ of heat. Which measurement (A or B) corresponds to conditions of constant pressure? Explain.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1387
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos