14. Solutions
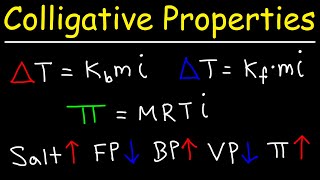
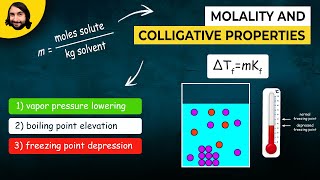
Freezing Point Depression

Get help from an AI Tutor
Ask a question to get started.
Problem 144
Textbook Question
Textbook QuestionElemental analysis of b@carotene, a dietary source of vitamin A, shows that it contains 10.51% H and 89.49% C. Dissolving 0.0250 g of b@carotene in 1.50 g of camphor gives a freezing- point depression of 1.17 °C. What are the molecular weight and formula of b@carotene? [Kf for camphor is 37.7 1°C kg2>mol.]
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
363
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos