17. Acid and Base Equilibrium
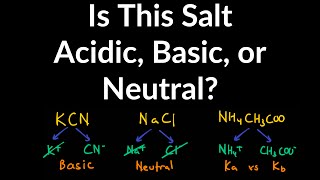
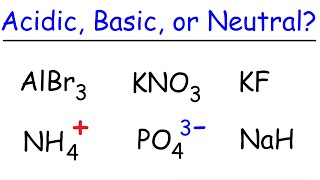
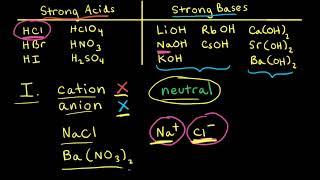
Ionic Salts
Problem 85
Textbook Question
Textbook QuestionAn unknown salt is either NaF, NaCl, or NaOCl. When 0.050 mol of the salt is dissolved in water to form 0.500 L of solution, the pH of the solution is 8.08. What is the identity of the salt?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
11mPlay a video:
850
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos