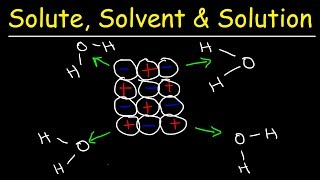
14. Solutions
Solutions: Solubility and Intermolecular Forces

Get help from an AI Tutor
Ask a question to get started.
Problem 22
Textbook Question
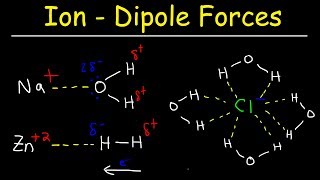
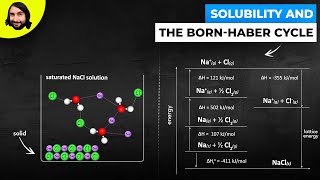
Textbook QuestionKBr is relatively soluble in water, yet its enthalpy of solution is + 19.8 kJ/mol. Which of the following statements provides the best explanation for this behavior? (a) Potassium salts are always soluble in water. (b) The entropy of mixing must be unfavorable. (c) The enthalpy of mixing must be small compared to the enthalpies for breaking up water–water interactions and K–Br ionic interactions. (d) KBr has a high molar mass compared to other salts like NaCl.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1812
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos