8. Thermochemistry
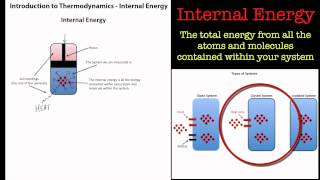
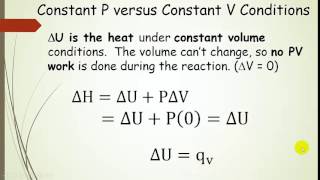
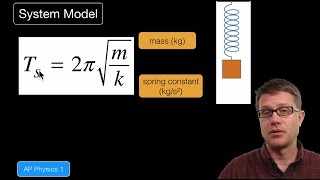
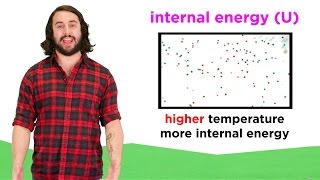
Internal Energy
Problem 4
Textbook Question
Textbook QuestionFor which of the following reactions are ΔE and ΔH equal? (a) CO2(g) + H2O(l) → H2CO (b) 2 NaHCO3 (s) → Na2CO3(s) + H2O(g) + CO2(g) (c) 2 H2(g) + O2(g) → 2 H2O(g) (d) CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
507
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos