7. Gases
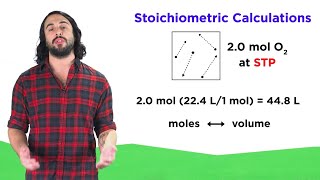
Gas Stoichiometry
Problem 55
Textbook Question
Textbook QuestionMagnesium can be used as a 'getter' in evacuated enclosures to react with the last traces of oxygen. (The magnesium is usually heated by passing an electric current through a wire or ribbon of the metal.) If an enclosure of 5.67 L has a partial pressure of O2 of 7.066 mPa at 30 °C, what mass of magnesium will react according to the following equation? 2 Mg1s2 + O21g2¡2 MgO1s2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
542
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos