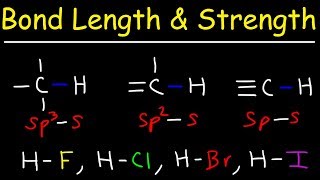11. Bonding & Molecular Structure
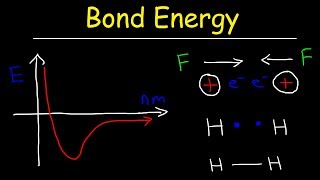
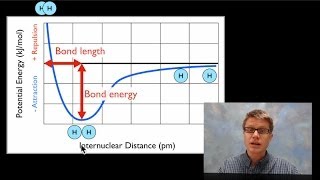
Bond Energy
Problem 84a
Textbook Question
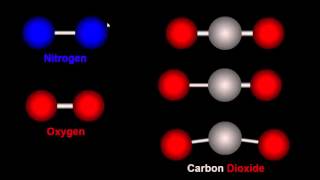
Textbook QuestionIn the Chemistry and the Environment box on free radicals in this chapter, we discussed the importance of the hydroxyl radical in reacting with and eliminating many atmospheric pollutants. However, the hydroxyl radical does not clean up everything. For example, chlorofluorocarbons—which destroy stratospheric ozone—are not attacked by the hydroxyl radical. Consider the hypothetical reaction by which the hydroxyl radical might react with a chlorofluorocarbon: OH( g) + CF2Cl2( g)¡HOF( g) + CFCl2( g) Use bond energies to explain why this reaction is improbable. (The C¬F bond energy is 552 kJ>mol.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
721
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos