Textbook Question
What is the density of lead in g/cm3 if a rectangular bar mea-suring 0.50 cm in height, 1.55 cm in width, and 25.00 cm in length has a mass of 220.9 g?
1122
views

 Verified step by step guidance
Verified step by step guidance
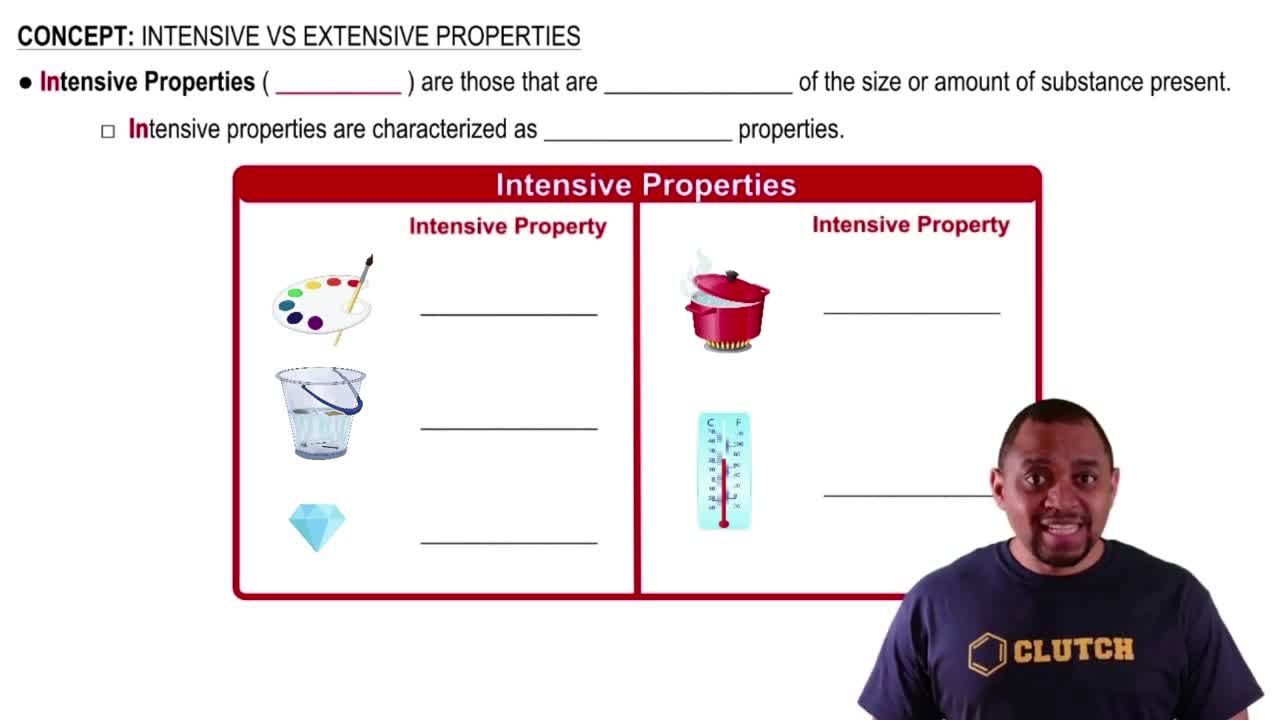
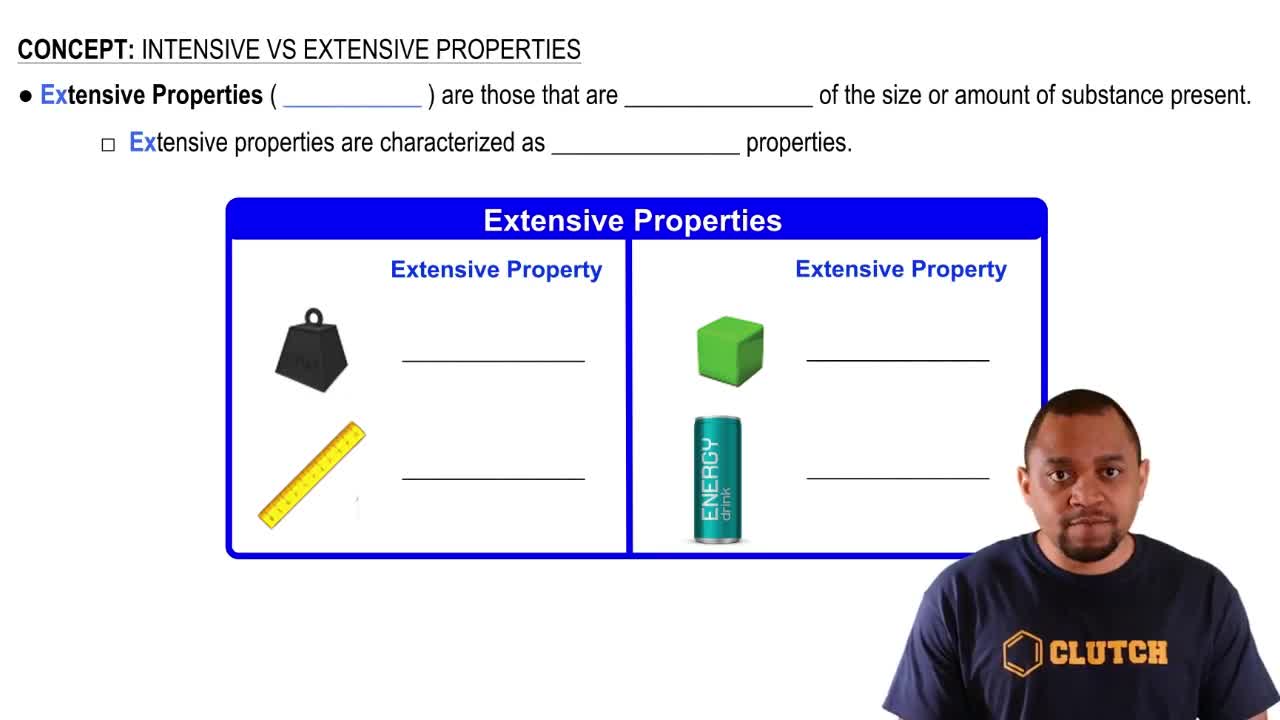

Which has more kinetic energy, a 1400 kg car moving at 115 km/h or a 12,000 kg truck moving at 38 km/h?