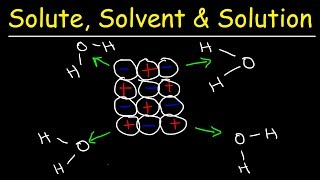
14. Solutions
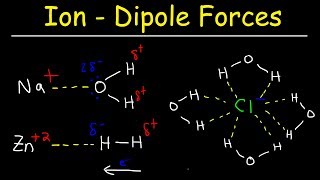
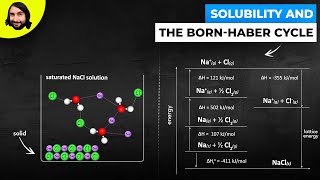
Solutions: Solubility and Intermolecular Forces
Problem 36d
Textbook Question
Textbook QuestionIndicate whether each statement is true or false: (c) The solubility of most gases in water decreases as the temperature increases because water is breaking its hydrogen bonding to the gas molecules as the temperature is raised.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
815
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos