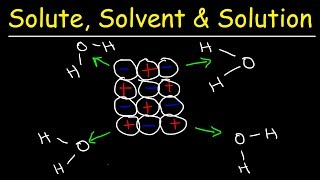
14. Solutions
Solutions: Solubility and Intermolecular Forces
Problem 16b
Textbook Question
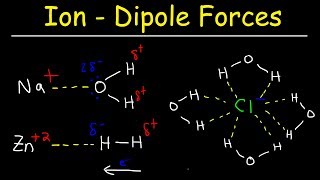
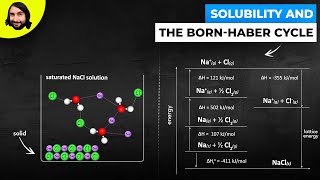
Textbook QuestionIndicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (b) CH2Cl2 in benzene 1C6H62
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
492
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos