13. Liquids, Solids & Intermolecular Forces
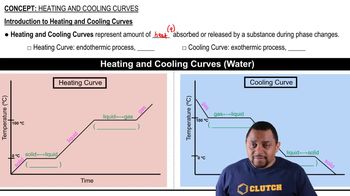
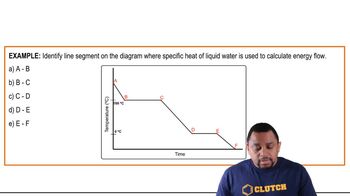
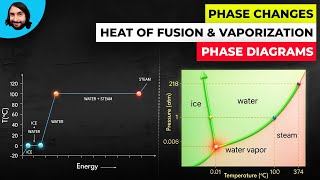
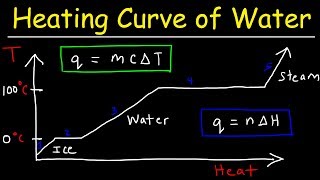
Heating and Cooling Curves
Problem 60
Textbook Question
Textbook QuestionSuppose that 1.15 g of rubbing alcohol (C3H8O) evaporates from a 65.0-g aluminum block. If the aluminum block is initially at 25 °C, what is the final temperature of the block after the evaporation of the alcohol? Assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 °C.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2025
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos