7. Gases
Standard Temperature and Pressure
Problem 77
Textbook Question
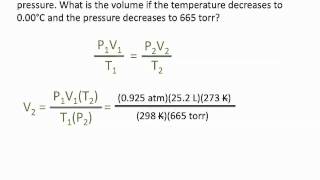
Textbook QuestionHydrogen gas (a potential future fuel) can be formed by the reaction of methane with water according to the equation: CH4( g) + H2O( g)¡CO( g) + 3 H2( g) In a particular reaction, 25.5 L of methane gas (measured at a pressure of 732 torr and a temperature of 25 °C) mixes with 22.8 L of water vapor (measured at a pressure of 702 torr and a temperature of 125 °C). The reaction produces 26.2 L of hydrogen gas at STP. What is the percent yield of the reaction?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
5043
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos