8. Thermochemistry
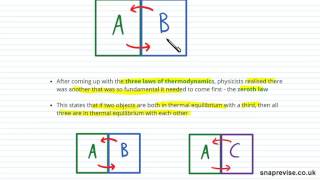
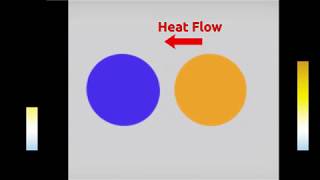
Thermal Equilibrium
Problem 68
Textbook Question
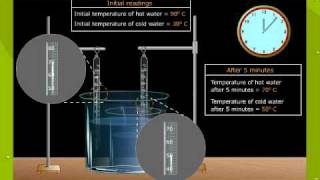
Textbook QuestionA 2.85-g lead weight, initially at 10.3 °C, is submerged in 7.55 g of water at 52.3 °C in an insulated container. What is the final temperature of both substances at thermal equilibrium?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2944
views
4
rank
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos