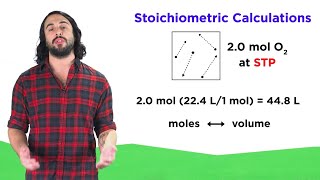
7. Gases
Gas Stoichiometry
Problem 136
Textbook Question
Textbook QuestionA group 3A metal has a density of 2.70 g>cm3 and a cubic unit cell with an edge length of 404 pm. Reaction of a 1.07 cm3 chunk of the metal with an excess of hydrochloric acid gives a colorless gas that occupies 4.00 L at 23.0 °C and a pressure of 740 mm Hg. (a) Identify the metal.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
345
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos