15. Chemical Kinetics
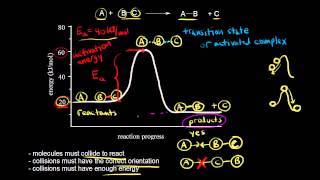
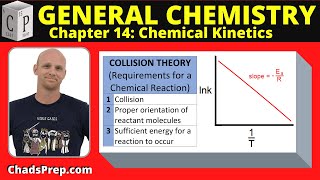
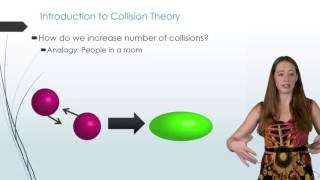
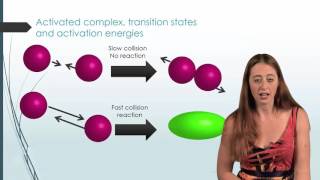
Collision Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 102b
Textbook Question
Textbook QuestionConsider the two reactions: O + N2¡NO + N Ea = 315 kJ>mol Cl + H2¡HCl + H Ea = 23 kJ>mol a. Why is the activation barrier for the first reaction so much higher than that for the second?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
408
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos