8. Thermochemistry
Constant-Volume Calorimetry
Problem 60
Textbook Question
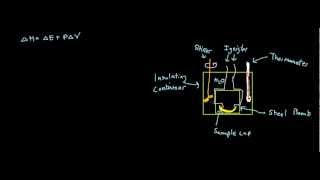
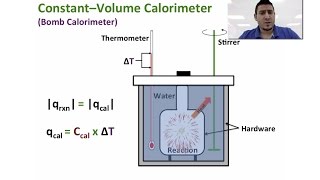
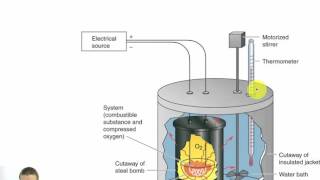
Textbook QuestionUnder constant-volume conditions, the heat of combustion of naphthalene 1C10H82 is 40.18 kJ>g. A 2.50-g sample of naphthalene is burned in a bomb calorimeter. The temperature of the calorimeter increases from 21.50 to 28.83 °C. (c) Suppose that in changing samples, a portion of the water in the calorimeter were lost. In what way, if any, would this change the heat capacity of the calorimeter?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1055
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos