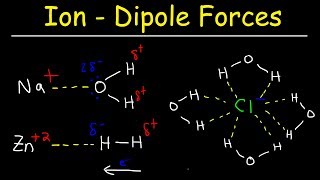
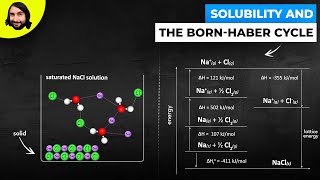
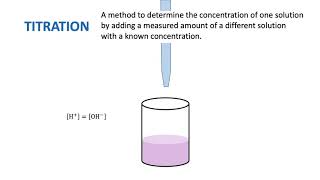
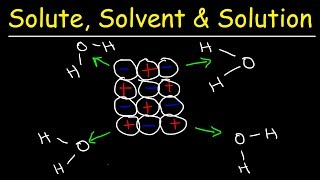
14. Solutions
Solutions: Solubility and Intermolecular Forces

Get help from an AI Tutor
Ask a question to get started.
Problem 21b
Textbook Question
Textbook QuestionTwo nonpolar organic liquids, hexane 1C6H142 and heptane 1C7H162, are mixed (b) Hexane and heptane are miscible with each other in all proportions. In making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure liquids?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
425
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos