10. Periodic Properties of the Elements
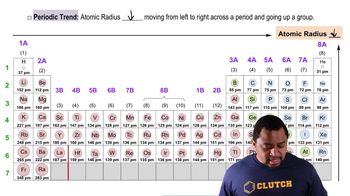
Periodic Trend: Atomic Radius
Problem 22
Textbook Question
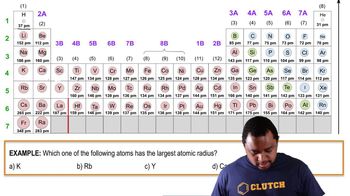
Textbook QuestionWhich of the following statements about the bonding atomic radii in Figure 7.7 is incorrect? (i) For a given period, the radii of the representative elements generally decrease from left to right across a period. (ii) The radii of the representative elements for the n = 3 period are all larger than those of the corresponding elements in the n = 2 period. (iii) For most of the representative elements, the change in radius from the n = 2 to the n = 3 period is greater than the change in radius from n = 3 to n = 4. (iv) The radii of the transition elements generally increase moving from left to right within a period. (v) The large radii of the Group 1 elements are due to their relatively small effective nuclear charges.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1109
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos