12. Molecular Shapes & Valence Bond Theory
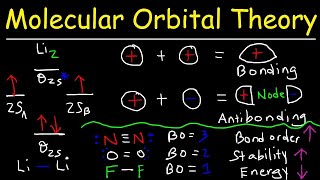
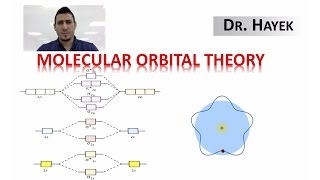
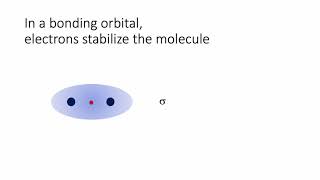
Molecular Orbital Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 56
Textbook Question
Textbook QuestionWrite orbital diagrams (boxes with arrows in them) to represent the electron configurations—without hybridization—for all the atoms in SF2. Circle the electrons involved in bonding. Draw a three-dimensional sketch of the molecule and show orbital overlap. What bond angle do you expect from the unhybridized orbitals? How well does valence bond theory agree with the experimentally measured bond angle of 98.2° ?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1165
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos