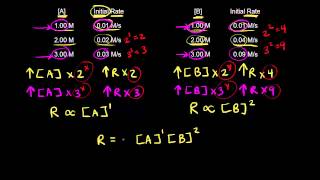15. Chemical Kinetics
Rate Law
Problem 37a
Textbook Question
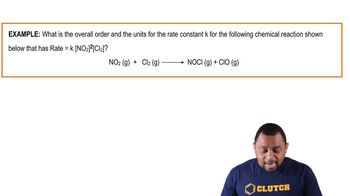
Textbook QuestionConsider the gas-phase reaction between nitric oxide and bromine at 273 C: 2 NO1g2 + Br21g2¡2 NOBr1g2. The following data for the initial rate of appearance of NOBr were obtained: Experiment 3no4 1M 2 3br2 4 1M 2 Initial Rate 1M,s2 1 0.10 0.20 24 2 0.25 0.20 150 3 0.10 0.50 60 4 0.35 0.50 735 (b) Calculate the average value of the rate constant for the appearance of NOBr from the four data sets.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
969
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos