14. Solutions
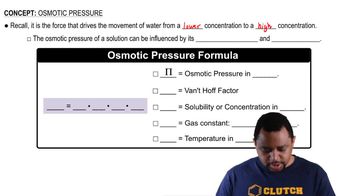
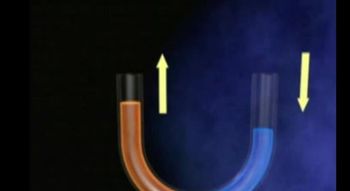
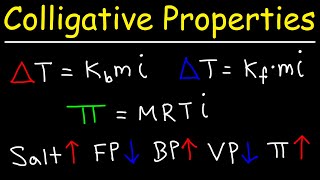
Osmotic Pressure

Get help from an AI Tutor
Ask a question to get started.
Problem 119
Textbook Question
Textbook QuestionWhat osmotic pressure in mm Hg would you expect for an aqueous solution of 11.5 mg of insulin 1mol. weight = 59902 in 6.60 mL of solution at 298 K? What would the height of the water column be in meters? The density of mercury is 13.534 g/mL at 298 K.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
560
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos