8. Thermochemistry
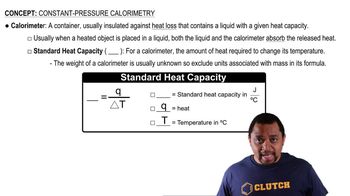
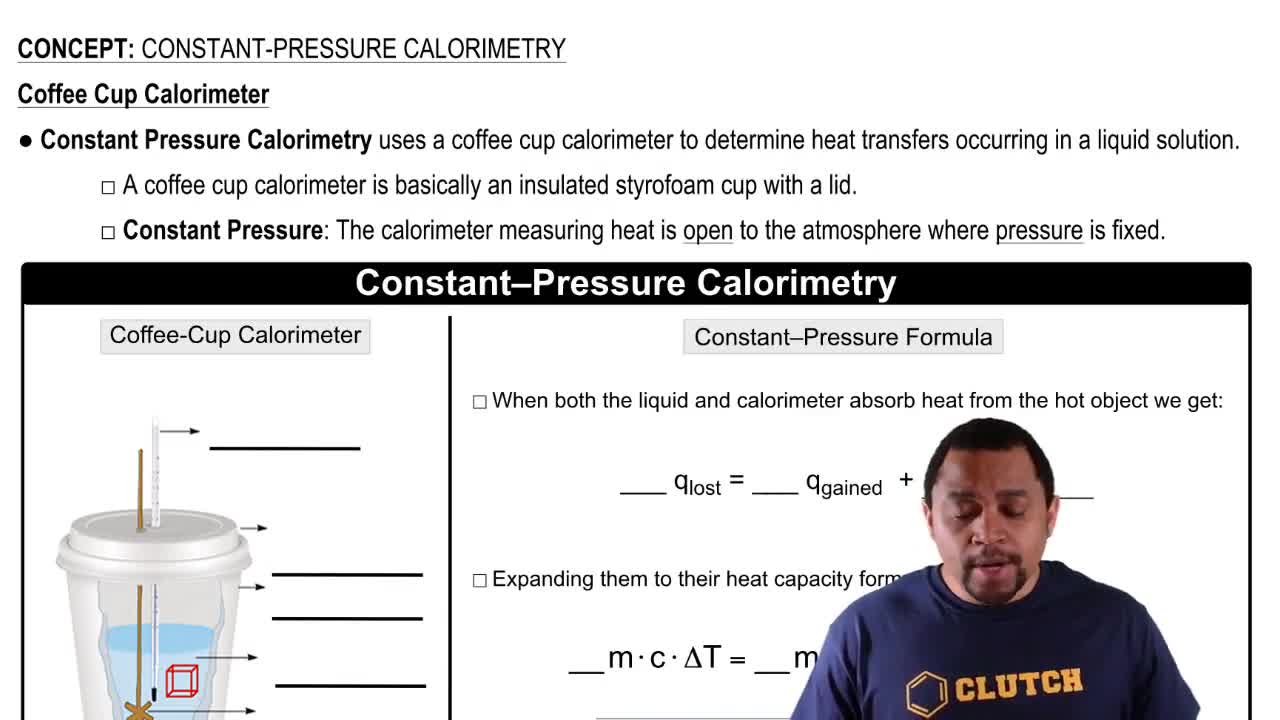
Constant-Pressure Calorimetry
Problem 106a
Textbook Question
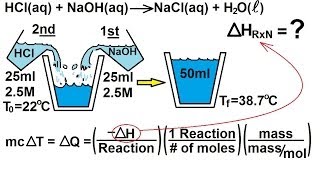
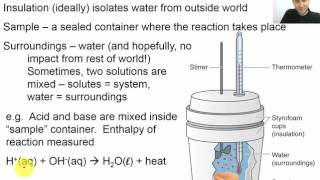
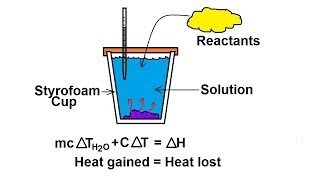
Textbook QuestionA coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.2 °C. A 200-g block of silver metal is heated to 100.5 °C by putting it in a beaker of boiling water. The specific heat of Ag(s) is 0.233J>1g # K2. The Ag is added to the calorimeter, and after some time the contents of the cup reach a constant temperature of 30.2 °C. (d) What would be the final temperature of the system if all the heat lost by the silver block were absorbed by the water in the calorimeter?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1106
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos