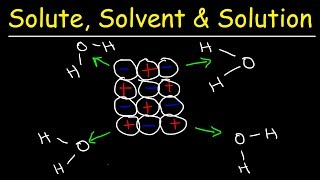
14. Solutions
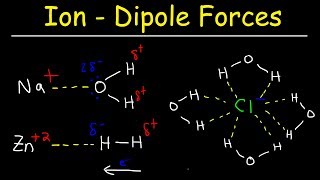
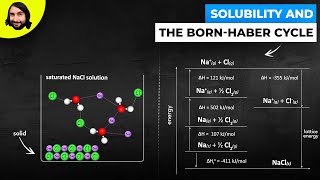
Solutions: Solubility and Intermolecular Forces

Get help from an AI Tutor
Ask a question to get started.
Problem 91
Textbook Question
Textbook QuestionThe 'free-base' form of cocaine (C17H21NO4) and its protonated hydrochloride form (C17H22ClNO4) are shown below; the free-base form can be converted to the hydrochloride form with one equivalent of HCl. For clarity, not all the carbon and hydrogen atoms are shown; each vertex represents a carbon atom with the appropriate number of hydrogen atoms so that each carbon makes four bonds to other atoms

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
938
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos