11. Bonding & Molecular Structure
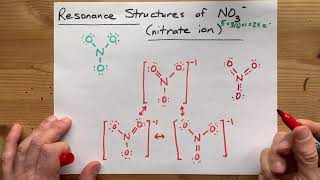
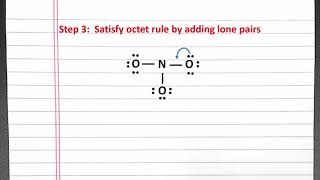
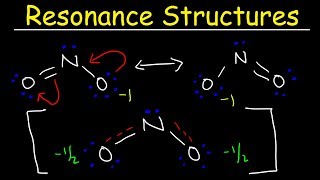
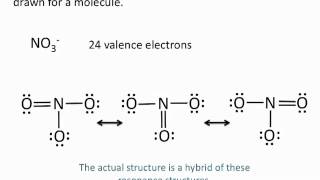
Resonance Structures
Problem 54
Textbook Question
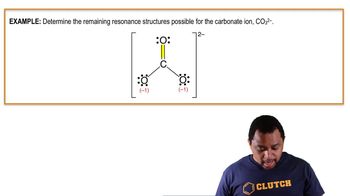
Textbook QuestionConsider the formate ion, HCO2-, which is the anion formed when formic acid loses an H+ ion. The H and the two O atoms are bonded to the central C atom. (b) Are resonance structures needed to describe the structure?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
1706
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos