13. Liquids, Solids & Intermolecular Forces
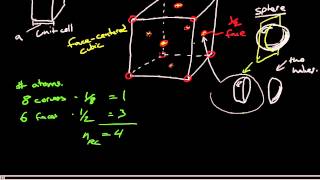
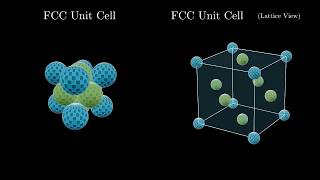
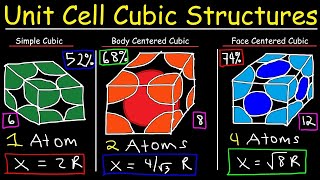
Face Centered Cubic Unit Cell
Problem 63
Textbook Question
Textbook QuestionCuI, CsI, and NaI each adopt a different type of structure. The three different structures are those shown in Figure 12.26. (a) Use ionic radii, Cs+ 1r = 1.81 A 2, Na+ 1r = 1.16 A 2, Cu+ 1r = 0.74 A 2, and, I- 1r = 2.06 A 2, to predict which compound will crystallize with which structure.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1191
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos