14. Solutions
Solutions: Solubility and Intermolecular Forces
Problem 3b
Textbook Question
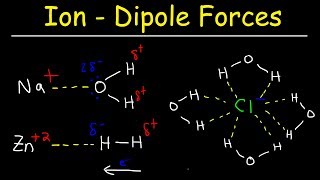
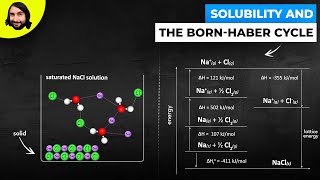
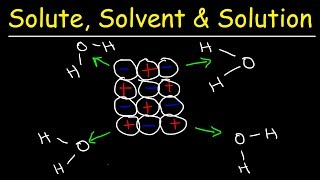
Textbook QuestionConsider two ionic solids, both composed of singly charged ions, that have different lattice energies. (b) If not, which solid will be more soluble in water, the one with the larger lattice energy or the one with the smaller lattice energy? Assume that solute–solvent interactions are the same for both solids. [Section 13.1]
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
405
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos