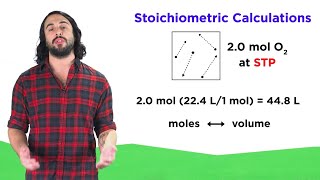
7. Gases
Gas Stoichiometry
Problem 126
Textbook Question
Textbook QuestionGaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I21s2 + 5 F21g2¡2 IF51g2 A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (a) What is the partial pressure of IF5 in the flask?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
961
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos