10. Periodic Properties of the Elements
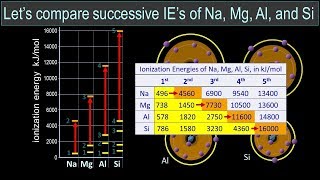
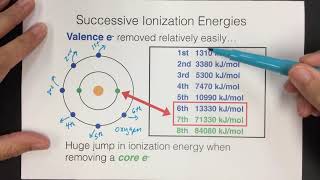
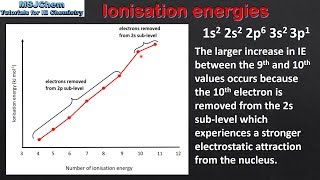
Periodic Trend: Successive Ionization Energies

Get help from an AI Tutor
Ask a question to get started.
Problem 120
Textbook Question
Textbook QuestionAt 50 °C, the ion-product constant for H2O has the value Kw = 5.48 * 10-14. (a) What is the pH of pure water at 50 °C? (b) Based on the change in Kw with temperature, predict whether ΔH is positive, negative, or zero for the autoionization reaction of water: 2 H2O1l2 Δ H3O+1aq2 + OH-1aq2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2212
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos