6. Chemical Quantities & Aqueous Reactions
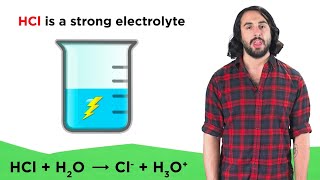
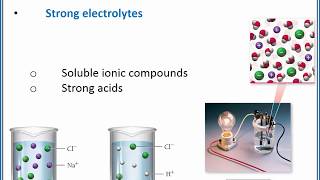
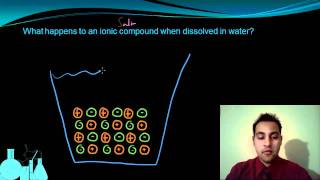
Electrolytes
Problem 20c
Textbook Question
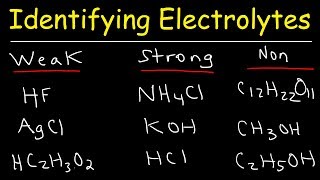
Textbook QuestionAcetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound? What solute particles are present in an aqueous solution of CH3COCH3?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1519
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos