11. Bonding & Molecular Structure
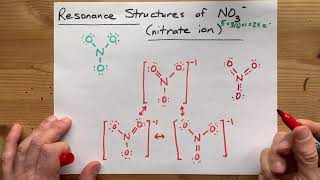
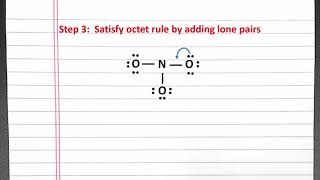
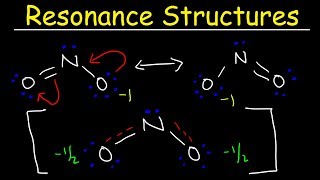
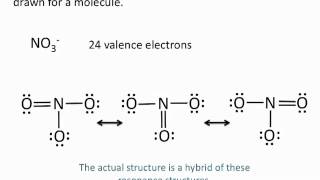
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 114
Textbook Question
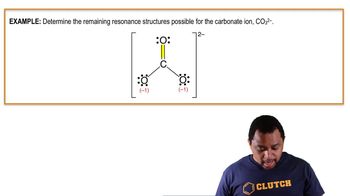
Textbook QuestionSulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur tetrafluoride monoxide 1OSF42 according to the following unbalanced reaction: SF41g2 + O21g2¡OSF41g2 The O atom and the four F atoms in OSF4 are bonded to a central S atom. (b) Write a Lewis structure of OSF4 in which the formal charges of all atoms are zero.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
208
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos