3. Chemical Reactions
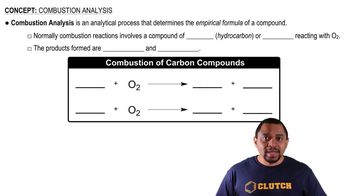
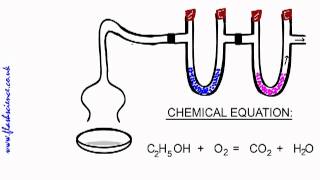
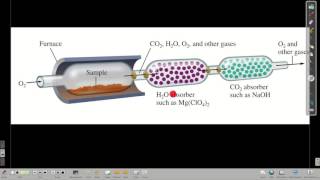
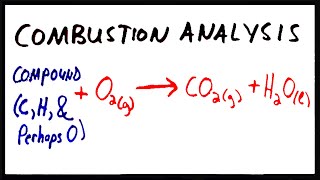
Combustion Analysis
Problem 96
Textbook Question
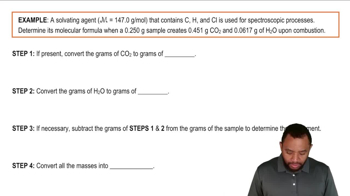
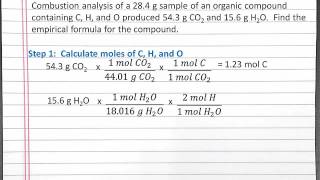
Textbook QuestionAn organic compound was found to contain only C, H, and Cl. When a 1.50-g sample of the compound was completely combusted in air, 3.52 g of CO2 was formed. In a separate experiment, the chlorine in a 1.00-g sample of the compound was converted to 1.27 g of AgCl. Determine the empirical formula of the compound.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
2554
views
2
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos