11. Bonding & Molecular Structure
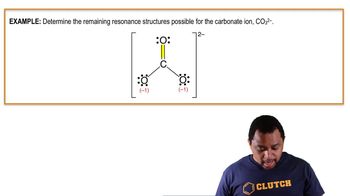
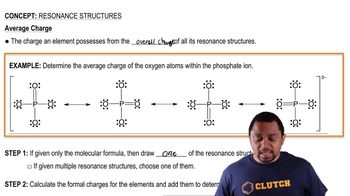
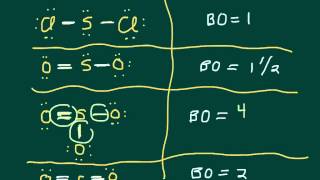
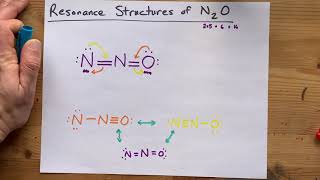
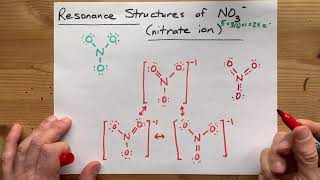
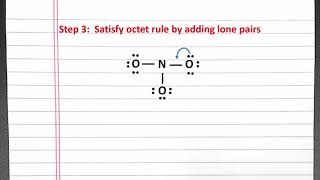
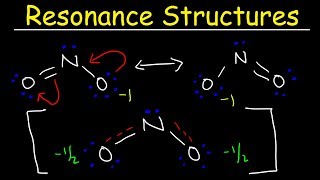
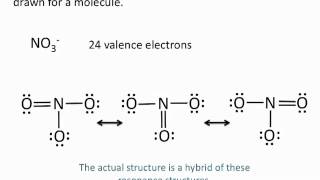
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 66
Textbook Question
Textbook Question(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe¬O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in part (a) yields the most favorable formal charges for the molecule?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1116
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos