8. Thermochemistry
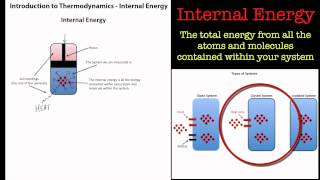
Internal Energy
Problem 65
Textbook Question
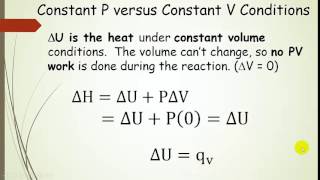
Textbook QuestionA reactiont akes place at a constant pressure of 1.10 atm with an internal energy change (ΔE) of 71.5 kJ and a volume decrease of 13.6 L. What is the enthalpy change (ΔH) for the reaction? (1 L-atm = 101.325 J)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1062
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos