10. Periodic Properties of the Elements
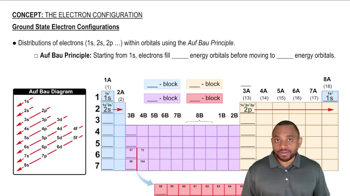
The Electron Configuration
Multiple Choice
Multiple ChoiceWhich of the following statements is false?
A
The sublevels of each principal level are not degenerate for multielectron atoms.
B
In a hydrogen atom, the sublevels of each principal level are degenerate.
C
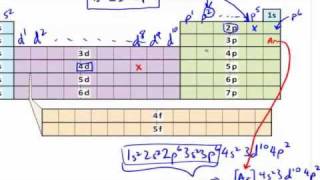
Due to shielding, the 3d orbitals lie lower in energy than the 4s orbital.
D
Some atoms have anomalies in their electron configurations due to the fact that energy separations of the orbitals become smaller as the principal quantum number increases.
239
views
Related Videos
Related Practice
Showing 1 of 15 videos