6. Chemical Quantities & Aqueous Reactions
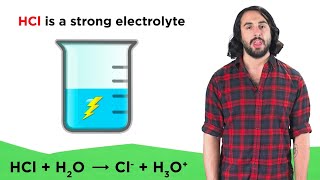
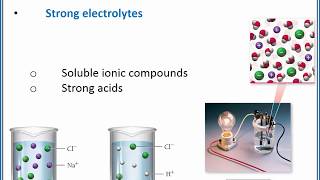
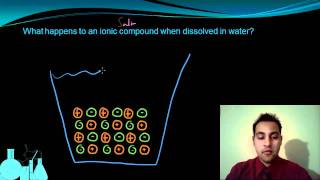
Electrolytes
Problem 36
Textbook Question
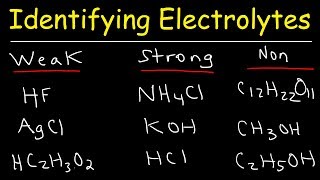
Textbook QuestionAn aqueous solution of an unknown solute is tested with litmus paper and found to be acidic. The solution is weakly conducting compared with a solution of NaCl of the same concentration. Which of the following substances could the unknown be: KOH, NH3, HNO3, KClO2, H3PO3, CH3COCH3 (acetone)?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1272
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos